Difference between Solid and Gas
Key difference: Solid, liquid, gas and plasma are the four primary states of matter in which objects can be found on Earth. Solid is a state of matter that has a fixed shape and fixed volume. A gas is a state of matter that has no definite shape or volume. Gases are either made up of one type of atom or compound molecules made from a variety of atoms.
 Solid, liquid, gas and plasma are the four primary states of matter in which objects can be found on Earth. Almost all substances can be found in either one of these four states. Water is the best example that can be used to describe states of matter as it can be found abundantly in three of the four states, ice (solid), water (liquid) and vapor (gas). While lighting or neon gases constitute as plasma.
Solid, liquid, gas and plasma are the four primary states of matter in which objects can be found on Earth. Almost all substances can be found in either one of these four states. Water is the best example that can be used to describe states of matter as it can be found abundantly in three of the four states, ice (solid), water (liquid) and vapor (gas). While lighting or neon gases constitute as plasma.
Solid is a state of matter that has a fixed shape and fixed volume. A solid is made up of tiny particles of matter such as atoms and molecules that are held together by chemical bonds. The atoms and molecules are held rigidly in place that does not allow the solid to change shape or volume. Hence, a solid cannot fill out the shape of a container like a liquid or fill the every space of a container like a gas.
The atoms and molecules in a solid are tightly bound to each other in either a regular geometric lattice or irregularly. The regular geometric lattice is most common and can be found in most solids, including ice. Solids with irregularly bound atoms are known as amorphous solids. This includes glass and polystyrene.
When some solids are heated, the energy from the heat is absorbed by the atoms. The atoms then get exited and start to move around. The bonds that held the atom in place tend to loosen, allowing the atoms to move further away from each other. Essentially, this is the process of melting. When the solid has melted thoroughly, it is then deemed to be a liquid.
A gas is a state of matter that has no definite shape or volume. Gases are either made up of one type of atom or compound molecules made from a variety of atoms. Unlike a liquid or solid, these atoms or molecules are not held together via strong bonds or attraction. Hence, the atoms and molecules move around freely with a lot of space between the particles.
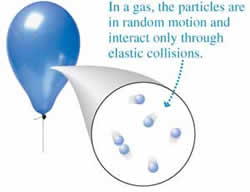 Due to this space between the molecules, most gases appear to be colorless to the naked eye. Hence, as compared to solid or liquid, it is very difficult to measure pressure, volume, number of particles and/or temperature of the gas. However, it is still possible.
Due to this space between the molecules, most gases appear to be colorless to the naked eye. Hence, as compared to solid or liquid, it is very difficult to measure pressure, volume, number of particles and/or temperature of the gas. However, it is still possible.
Furthermore, the ability of the atoms and molecules of the gas to spread out from each other allows a gas to fill the entire space of the container it is in. Scientists have been able to use this ability to measure weight and volume of the gas. Compared to the other states of matter, gases have low density and viscosity. Also, pressure and temperature influence the particles within a certain volume of gas. This means that the weight, density, and volume may fluctuate depending on the pressure and temperature.
As the pressure increases and temperature decreases, the molecules in the gas will come closer to each other. After a point, the molecules will be so close to each other that their bonds will allow them to become a liquid. Whereas, if the pressure decreases and the temperature increases, then the molecules will be forced to move further apart from each other and may even break their bonds altogether after a point.
Image Courtesy: cmapspublic.ihmc.us, szkomsk.edu.glogster.com







Comments
Smug
Tue, 11/28/2017 - 16:18
Vannesa Martinez
Fri, 12/11/2015 - 03:15
Great work
Brenda Marian d...
Fri, 04/11/2014 - 06:09
Great
Brenda Marian d...
Tue, 04/08/2014 - 07:39
Add new comment