Difference between Liquid and Gas
Key difference: Solid, liquid, gas and plasma are the four primary states of matter in which objects can be found on Earth. Liquid is a state of matter that has no fixed shape but has a definite volume. A gas is a state of matter that has no definite shape or volume. Gases are either made up of one type of atom or compound molecules made from a variety of atoms.
 Solid, liquid, gas and plasma are the four primary states of matter in which objects can be found on Earth. Almost all substances can be found in either one of these four states. Water is the best example that can be used to describe states of matter as it can be found abundantly in three of the four states, ice (solid), water (liquid) and vapor (gas). While lighting or neon gases constitute as plasma.
Solid, liquid, gas and plasma are the four primary states of matter in which objects can be found on Earth. Almost all substances can be found in either one of these four states. Water is the best example that can be used to describe states of matter as it can be found abundantly in three of the four states, ice (solid), water (liquid) and vapor (gas). While lighting or neon gases constitute as plasma.
Liquid is a state of matter that has no fixed shape but has a definite volume. Liquid is made up of tiny particles of matter such as atoms and molecules that are held together by chemical bonds. Liquid shares many of its characteristics with the solid and gaseous states. Such as similar to a gas it is free-flowing and can take the shape of the container it is placed in, however unlike a gas it cannot fill every space of the container. The density of liquid is closer to solid than gas and both are termed as condensed matter. A distinctive property of the liquid state is the surface tension that results in wetting objects when dipped into it.
Liquid particles are bounded firmly but not rigidly, which gives it the ability to flow. They are also able to move around one another freely, with limited particle mobility. The transformation of liquid to other states has to do with its molecules; as liquid is heated the molecules increase in vibrations and movement causing them to create greater distances between them. As the distance increases, after a while the liquid turns into a gas. During solidification, as the liquid is cooled, the molecules come together and form a specific order, known as crystallizing. The bonds between them become more rigid and stronger, and eventually come together to become a solid. Water is the most abundant liquid on the Earth and is considered a necessity for sustenance of life.
A gas is a state of matter that has no definite shape or volume. Gases are either made up of one type of atom or compound molecules made from a variety of atoms. Unlike a liquid or solid, these atoms or molecules are not held together via strong bonds or attraction. Hence, the atoms and molecules move around freely with a lot of space between the particles.
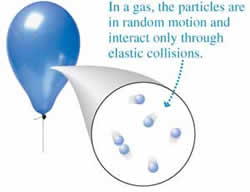 Due to this space between the molecules, most gases appear to be colorless to the naked eye. Hence, as compared to solid or liquid, it is very difficult to measure pressure, volume, number of particles and/or temperature of the gas. However, it is still possible.
Due to this space between the molecules, most gases appear to be colorless to the naked eye. Hence, as compared to solid or liquid, it is very difficult to measure pressure, volume, number of particles and/or temperature of the gas. However, it is still possible.
Furthermore, the ability of the atoms and molecules of the gas to spread out from each other allows a gas to fill the entire space of the container it is in. Scientists have been able to use this ability to measure weight and volume of the gas. Compared to the other states of matter, gases have low density and viscosity. Also, pressure and temperature influence the particles within a certain volume of gas. This means that the weight, density, and volume may fluctuate depending on the pressure and temperature.
As the pressure increases and temperature decreases, the molecules in the gas will come closer to each other. After a point, the molecules will be so close to each other that their bonds will allow them to become a liquid. Whereas, if the pressure decreases and the temperature increases, then the molecules will be forced to move further apart from each other and may even break their bonds altogether after a point.
Image Courtesy: whs.wsd.wednet.edu, szkomsk.edu.glogster.com









Add new comment